Cassiopea’s Breakthrough Acne Treatment
I have covered Italian company Cassiopea numerous times on this blog. Primarily due to its Breezula topical product for male pattern hair loss. Earlier this year, Cassiopea announced very good 12-month Phase 2 clinical trial results for Breezula.
Clascoterone, DHT and Acne
Cassiopea is also working on an acne product called Winlevi. Both Breezula and Winlevi are based on the same key ingredient: Clascoterone. However, the dosage in Winlevi is significantly lower at 1%. Winlevi is almost ready to come to market per Cassiopea’s product pipeline.
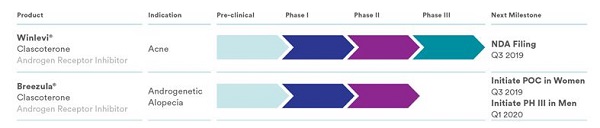
Earlier this week, Cassiopea made a major announcement. The company has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking marketing approval for clascoterone cream 1% for the treatment of acne.
Some interesting points in the press release and my thoughts:
- Clascoterone cream 1% targets androgen receptors at the site of application. This inhibits the local (skin) effects of dihydrotestosterone (DHT). Apparently, DHT is a key driver of acne lesion development. Most people wrongly assume it is solely testosterone.
- This acne treatment product will be safe to use in both males and females.
- If approved, clascoterone cream 1% will represent the first new mechanism of action in the treatment of acne in almost 40 years. Interestingly, if Breezula is approved for hair loss in 2021, it will be the first new topical or oral treatment for hair loss in almost 25 years.
The post Cassiopea’s Breakthrough Acne Treatment appeared first on Hair Loss Cure 2020.

